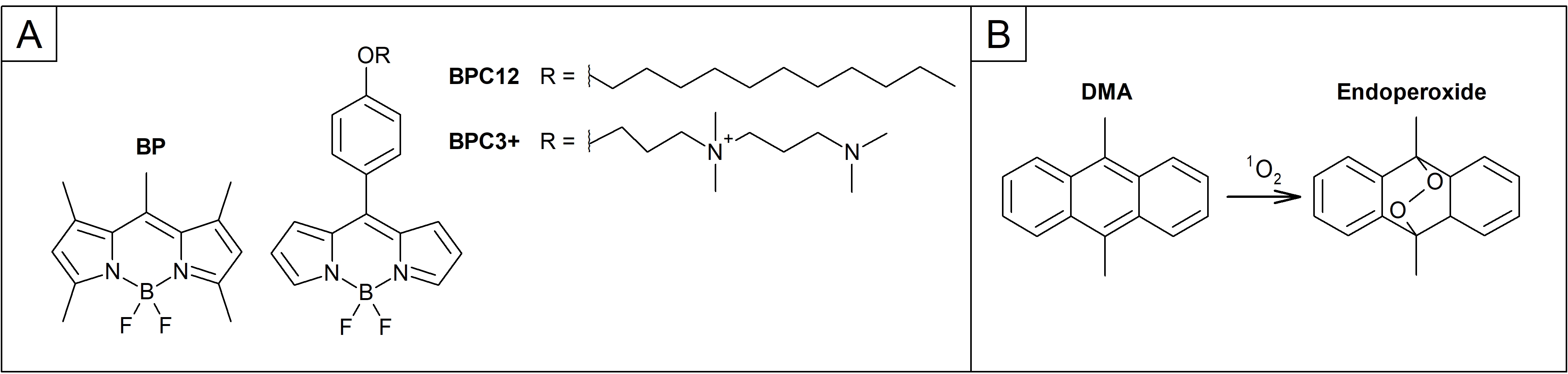
Chart 1. Molecular structures of A) the BODIPY derivatives; BP, BPC12 and BPC3+. B) A reaction of the DMA with a singlet oxygen (1O2) to form the endoperoxide compound.
In this study, we investigate three different BODIPY derivatives of which two are molecular rotors (BPC12 and BPC3+, Chart 1A) and one is not (BP, Chart 1A). The phototoxic nature of all the dyes was examined with following studies:
- Absorption and fluorescence spectra in ethanol (EtOH), dimethyl sulfoxide (DMSO), toluene and polyethylene glycol (200 g/mol, PEG-200).
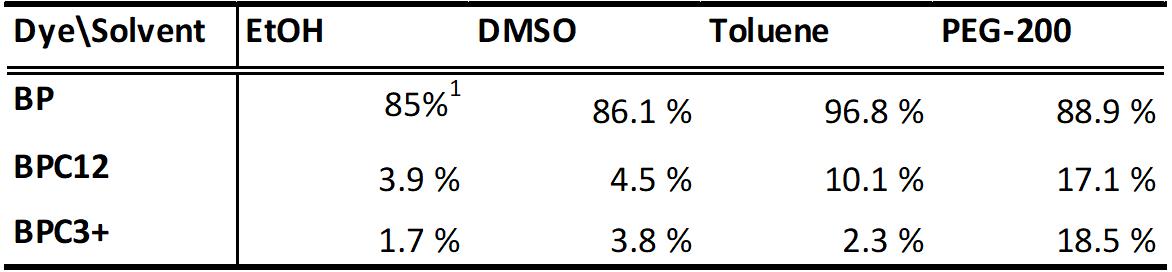
- Fluorescence quantum yields in EtOH, DMSO, toluene and PEG-200 by using BP in EtOH as a reference.
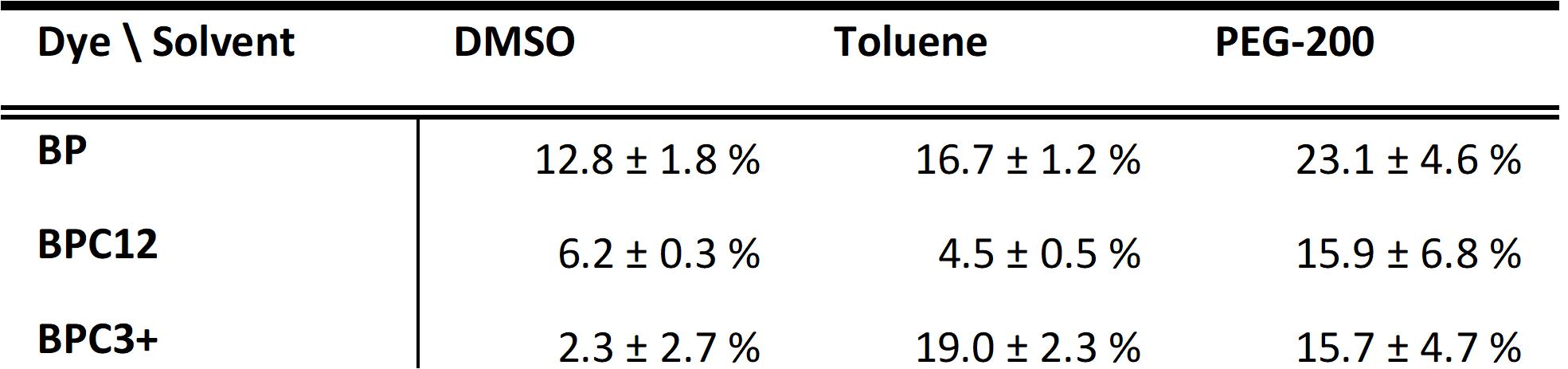
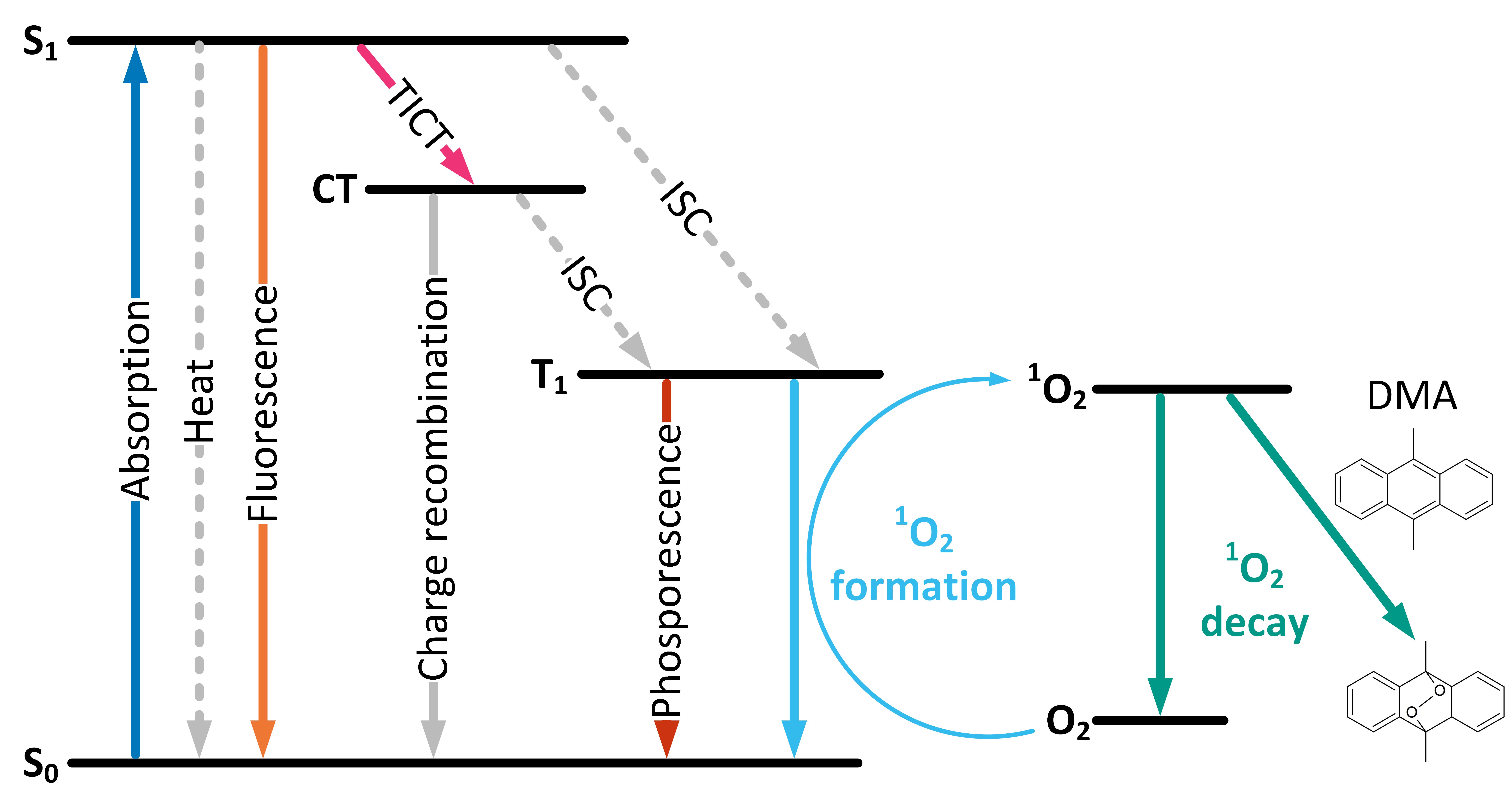
- Singlet oxygen quantum yields in DMSO, toluene and PEG-200 by using 2-(2,4,5,7-tetrabromo-6-oxido-3-oxo-3H-xanthen-9-yl)benzoate (Eosin Y) in DMSO as a reference and dimethyl anthracene (DMA, Chart 1B) as a singlet oxygen trap.
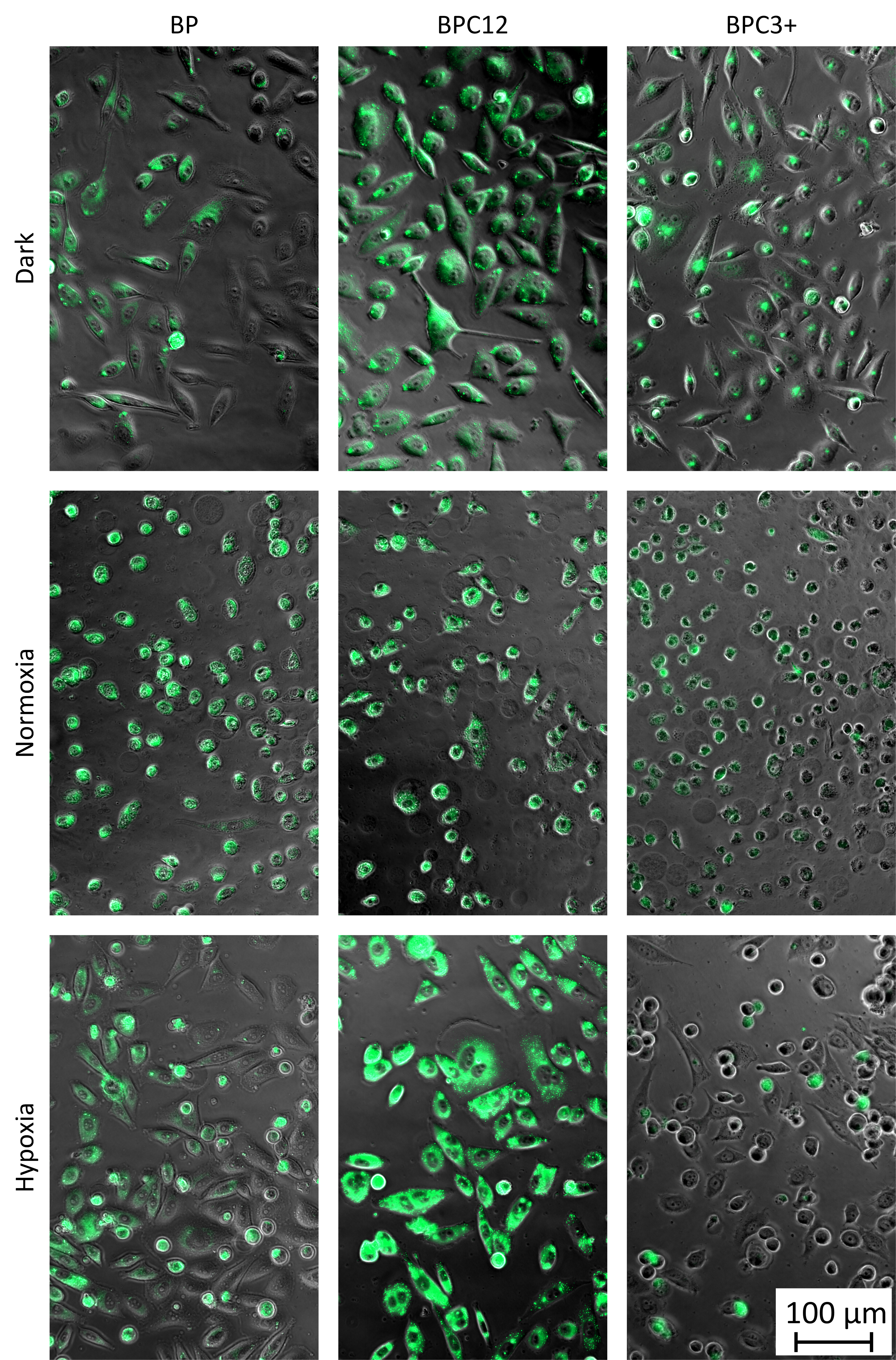
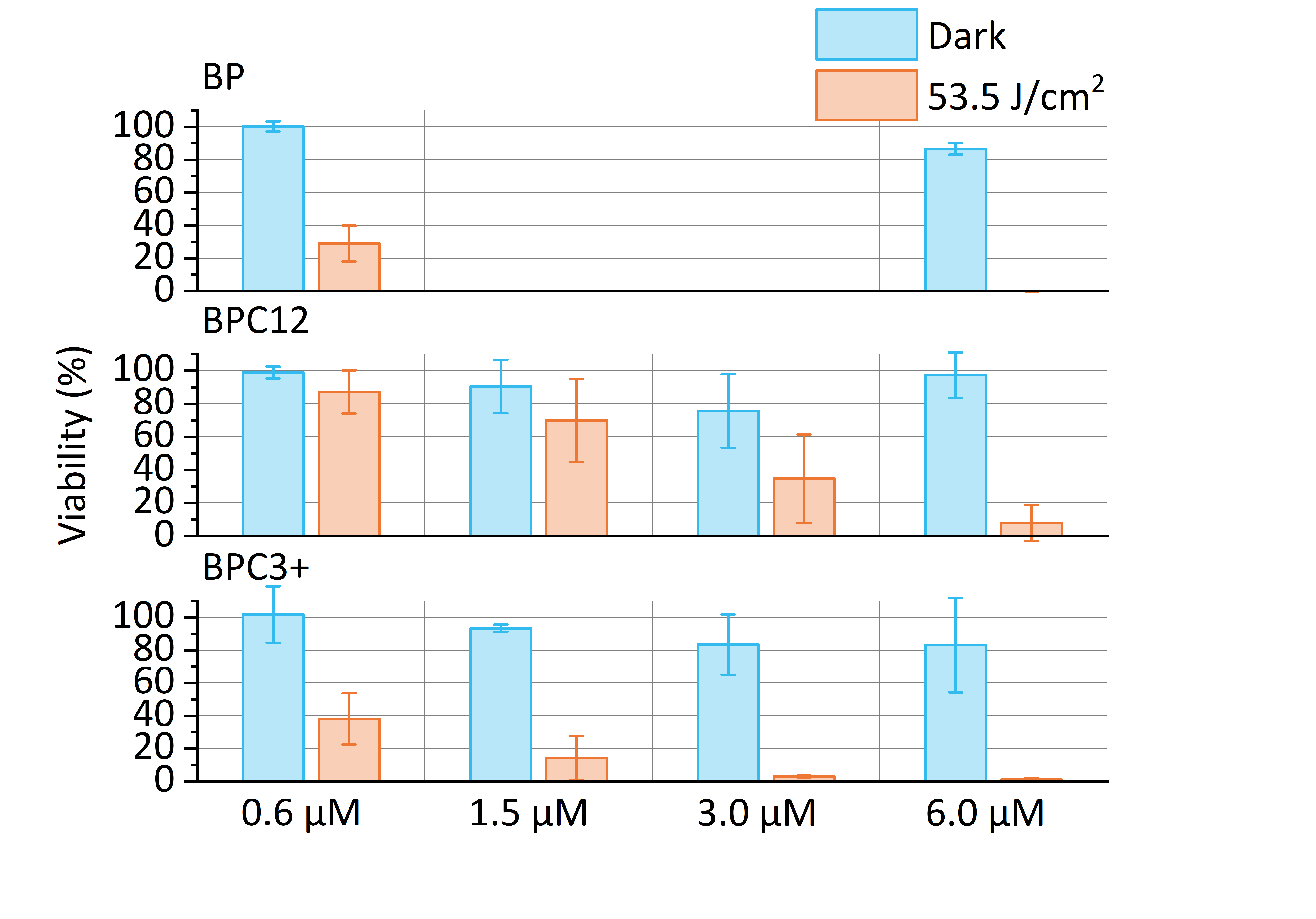
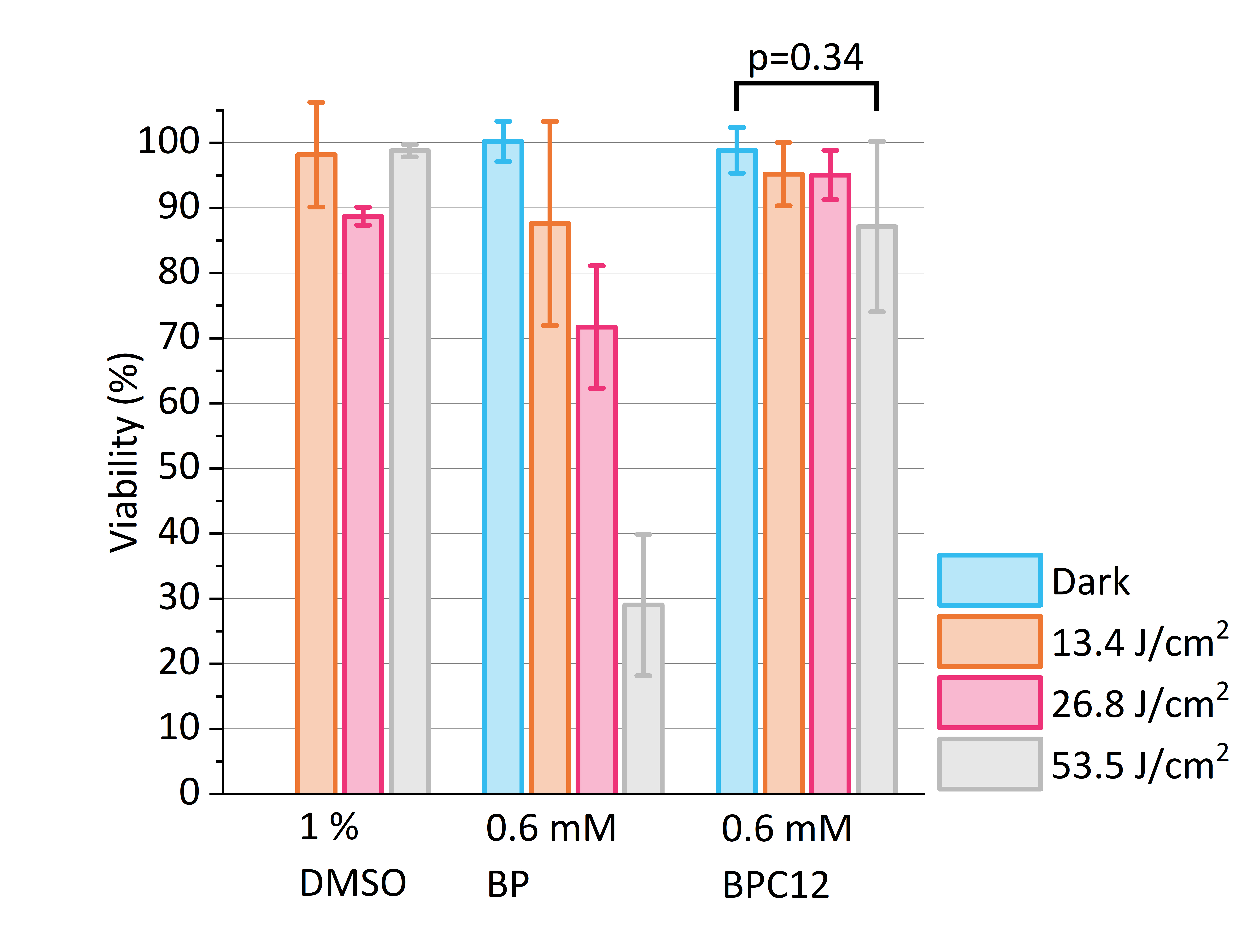
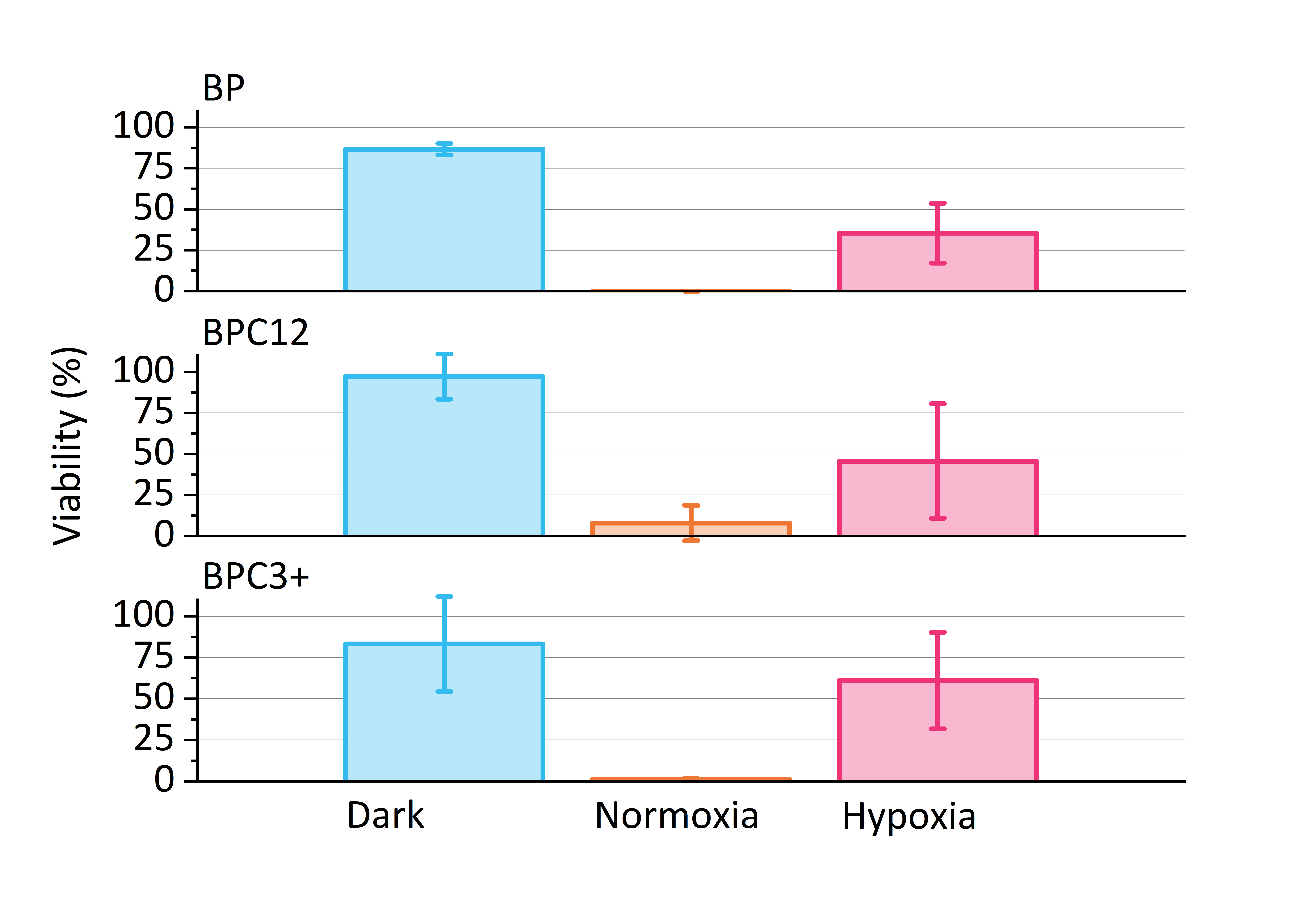
- The morphology changes and viabilities of human prostate cancer cell line (PC-3) in four different concentrations (0.6, 1.5, 3.0 and 6.0 μM), three different light dosages (13.4, 26.8 and 53.5 J/cm2) and two different oxygen levels (normoxia: ≈20 %; hypoxia: ≈0.08 %).